Change of State
Change of State: Overview
This Topic covers sub-topics such as Condensation, Latent Heat, Latent Heat of Fusion, Difference between Evaporation and Boiling, Solidification, Phase Diagram of Water, Change of State, Vapourization, Latent Heat of Sublimation and, Normal Boiling Point
Important Questions on Change of State
The latent heat of fusion of a substance is always less than the latent heat of vaporization or latent heat of sublimation of the same substance. Explain.
Which of these changes can be reversed?
How much heat is required to convert of ice at to water at ? (Given specific heat of ice , specific heat of water , Latent heat of fusion )
of steam at is passed into a large block of ice at the mass of ice that melts is
Why does the cloud not getting frozen at very high height?
Name the type of vaporization that occurs on the surface of a liquid as it changes into a gas.
The phenomenon of _____ gives a cooling effect to the remaining liquid.
What happens in the process of solidification?
What is normal melting point?
What is the difference between evaporation and boiling?
Define triple point of a substance.
Describe the dependence of boiling point of a liquid on pressure.
List the differences between boiling and evaporation.
Define the term vaporisation with an example.
The boiling point increases with a decrease in pressure.
Bubbling effect is not visible in evaporation.
Define the boiling point of substance.
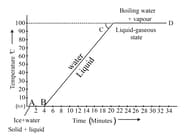
Identify the boiling point of water from the given temperature versus time graph for heating of ice.